
In September 2023, to mitigate environmental risks and impacts from the intentional use of microplastics, the European Chemicals Agency (ECHA) implemented restrictions on products containing intentionally added microplastics. ECHA has outlined specific obligations and constraints for cosmetic companies using microplastics to manufacture their finished products and has also specified when such products could be banned from the market.
Seppic as a supplier of ingredients helps to better understand the microplastic restriction and to find the right solution to replace rheology modifiers that are microplastic.
How can microplastics be identified according to the European microplastics restriction?
According to Regulation (EU) 2023/2055, issued by the European Commission on September 25, 2023, microplastics also called Synthetic Polymer Microparticles (SPM) are officially defined as:
● solid polymers particles with a size less than 5 mm
● except if they are biodegradable or natural or soluble in water or without carbon atoms
The solubility criterion is essential in determining whether a synthetic polymer qualifies as an SPM. According to Appendix 16, the solubility test must be conducted at pH 7 on a test material that is compositionally comparable to the polymer particles in their commercialized form.
On the one hand, there are solid synthetic polymers that require neutralization during formulation, resulting at pH 7 in a form that differs from their supplied state. For these polymers, the solubility test is not applicable, which prevents them from being excluded from the microplastic definition based on the solubility criterion. As a result, these polymers, such as carbomer, are defined as microplastics.
On the other hand, there are polymers that are supplied in a pre-neutralized form, meaning they remain unchanged at pH 7 from their supplied state. It is the case of Seppic’s solid synthetic polymers. For these polymers, the solubility test can be conducted under the conditions specified by the restriction. According to measurements made following OECD 120 guidelines, Seppic’s synthetic solid polymers are non-microplastic based on their solubility test results.
What is at stake for cosmetics manufacturers using microplastics?
1. What are the obligations?
Cosmetics manufacturers using microplastics in their products, such as polymers requiring neutralization during formulation, must navigate complex steps to ensure regulatory compliance
Firstly, cosmetic manufacturers are required to report annually on the use of microplastics at their production sites.
Secondly, cosmetic manufacturers must demonstrate that any microplastic used is permanently modified during formulation; if this condition is met, the formulation falls outside the scope of the restriction.
If not, thirdly, they must show that the microplastic is permanently modified during its intended end use. In this case, the formulation qualifies for a derogation and must include information stating how to avoid release into the environment, along with meeting reporting requirements.
If neither of these conditions is met, the formulation is banned from the market.
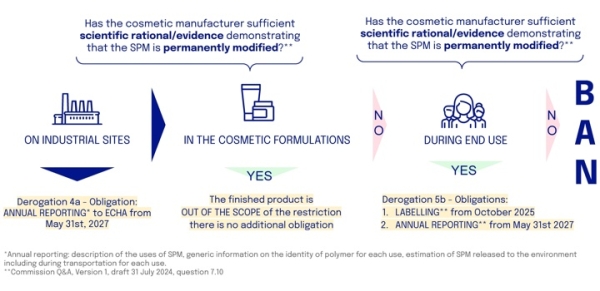
Cosmetic manufacturers obligations linked to the use of microplastics
As Seppic synthetic solid rheology modifiers are all excluded from the microplastic definition, using them allows formulators to confidently leverage their properties without concern for microplastic restrictions.
2. When will cosmetic products containing microplastics be banned?
If there is lack of evidence to support the permanent modification of a microplastic ingredient in a cosmetic product and during the end use, the said product is prohibited from the market. The date of the market ban depends on its use. Here are the three dates to remember:
● From October 2027: rinse-off products
● From October 2029: leave-on products
● From October 2035: lip, nail and make-up products (with labeling on the cosmetic product from October 2031)
How to replace synthetic solid rheology modifiers that are microplastic ?
In cosmetic products, rheology modifiers are essential to bring stability and viscosity while presenting sensorial benefits. However, some of these rheology modifiers are microplastic such as carbomer (INCI) – which is one of the most used rheology modifiers in cosmetics worldwide. According to Mintel, on the European market, more than 1 500 new cosmetic products containing carbomer are launched per year in skin care products.
What non-microplastic alternatives are available to replace microplastic rheology modifiers, such as carbomer, while offering an even better performance or other benefits?
In skin care or hygiene products containing DHA or presenting a high acidic pH, while carbomer is respectively not compatible or not able to thicken, Seppic non microplastic solid rheology modifiers such as Sepinov™ EMT 10, Sepinov™ WEO and Sepimax Zen™ achieve excellent performance in these applications. Sepinov™ WEO is specifically recommended for EO free concepts.
For classic emulsions or even gel creams that could contain a high percentage of oils, they also present a better oil stabilizing performance in comparison with carbomer.
Moreover, some cosmetic products contain solid particles in suspension. It is necessary to have a rheology modifier which presents an appropriate rheological profile and the right suspending effect. It is the case of Sepimax Zen™ that allows a suspension of particles even at a very low viscosity, better than carbomer.
In addition, with products containing electrolytes, Sepimax Zen™ presents an extreme resistance to them that allows to support the formulation, it offers a chassis effect, which is not the case of carbomer.
To conclude, formulating with Seppic’s rheology modifiers means formulating with ease of mind, thanks to:
● Non microplastic status proven.
● In-depth expertise on the microplastic restriction.
● Reactivity when full folder of proof is requested by the competent authorities.
● No constraints nor obligations for cosmetic manufacturers linked to the microplastic restriction.
● High technical performance in formulation.
